Lineage factors and differentiation states in lung cancer progression
Lung cancer encompasses a heterogeneous group of malignancies. Here we discuss how the remarkable diversity of major lung cancer subtypes is manifested in their transforming cell of origin, oncogenic dependencies, phenotypic plasticity, metastatic competence and response to therapy. More specifically, we review the increasing evidence that links this biological heterogeneity to the deregulation of cell lineage-specific pathways and the transcription factors that ultimately control them. As determinants of pulmonary epithelial differentiation, these poorly characterized transcriptional networks may underlie the etiology and biological progression of distinct lung cancers, while providing insight into innovative therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
265,23 € per year
only 5,30 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Aggressive early-stage lung adenocarcinoma is characterized by epithelial cell plasticity with acquirement of stem-like traits and immune evasion phenotype
Article 25 June 2021

Spatial transcriptomics delineates molecular features and cellular plasticity in lung adenocarcinoma progression
Article Open access 19 September 2023
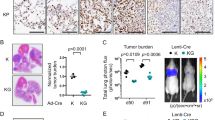
Tumor progression and chromatin landscape of lung cancer are regulated by the lineage factor GATA6
Article Open access 10 March 2020
References
- Travis WD, Brambilla E, Riely GJ . New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol 2013; 31: 992–1001. CASGoogle Scholar
- Siegel R, Ma J, Zou Z, Jemal A . Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29. Google Scholar
- Morrisey EE, Hogan BL . Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell 2010; 18: 8–23. CASGoogle Scholar
- Desai TJ, Brownfield DG, Krasnow MA . Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 2014; 507: 190–194. CASGoogle Scholar
- Treutlein B, Brownfield DG, Wu AR, Neff NF, Mantalas GL, Espinoza FH et al. Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature 2014; 509: 371–375. CASGoogle Scholar
- Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 2013; 123: 3025–3036. CASGoogle Scholar
- Zuo W, Zhang T, Wu DZ, Guan SP, Liew AA, Yamamoto Y et al. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature 2015; 517: 616–620. CASGoogle Scholar
- Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 2015; 517: 621–625. ArticleCASGoogle Scholar
- Meuwissen R, Berns A . Mouse models for human lung cancer. Genes Dev 2005; 19: 643–664. CASGoogle Scholar
- Rawlins EL, Perl AK . The a'MAZE"ing world of lung-specific transgenic mice. Am J Respir Cell Mol Biol 2012; 46: 269–282. CASGoogle Scholar
- Blanpain C . Tracing the cellular origin of cancer. Nat Cell Biol 2013; 15: 126–134. CASGoogle Scholar
- Hanna JM, Onaitis MW . Cell of origin of lung cancer. J Carcinog 2013; 12: 6. CASGoogle Scholar
- Sutherland KD, Berns A . Cell of origin of lung cancer. Mol Oncol 2010; 4: 397–403. Google Scholar
- Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK . Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 2014; 14: 535–546. CASGoogle Scholar
- van Meerbeeck JP, Fennell DA, De Ruysscher DK . Small-cell lung cancer. Lancet 2011; 378: 1741–1755. Google Scholar
- Peifer M, Fernández-Cuesta L, Sos ML, George J, Seidel D, Kasper LH et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 2012; 44: 1104–1110. CASGoogle Scholar
- Meuwissen R, Linn SC, Linnoila RI, Zevenhoven J, Mooi WJ, Berns A . Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell 2003; 4: 181–189. CASGoogle Scholar
- Sutherland KD, Proost N, Brouns I, Adriaensen D, Song JY, Berns A . Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell 2011; 19: 754–764. CASGoogle Scholar
- Park KS, Liang MC, Raiser DM, Zamponi R, Roach RR, Curtis SJ et al. Characterization of the cell of origin for small cell lung cancer. Cell Cycle 2011; 10: 2806–2815. CASGoogle Scholar
- Song H, Yao E, Lin C, Gacayan R, Chen MH, Chuang PT . Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc Natl Acad Sci USA 2012; 109: 17531–17536. CASGoogle Scholar
- McFadden DG, Papagiannakopoulos T, Taylor-Weiner A, Stewart C, Carter SL, Cibulskis K et al. Genetic and clonal dissection of murine small cell lung carcinoma progression by genome sequencing. Cell 2014; 156: 1298–1311. CASGoogle Scholar
- Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P et al. LKB1 modulates lung cancer differentiation and metastasis. Nature 2007; 448: 807–810. CASGoogle Scholar
- Xiao Z, Jiang Q, Willette-Brown J, Xi S, Zhu F, Burkett S et al. The pivotal role of IKKalpha in the development of spontaneous lung squamous cell carcinomas. Cancer Cell 2013; 23: 527–540. CASGoogle Scholar
- Cancer Genome Atlas Research N. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012; 489: 519–525. Google Scholar
- Xu C, Fillmore CM, Koyama S, Wu H, Zhao Y, Chen Z et al. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell 2014; 25: 590–604. CASGoogle Scholar
- Quinlan MP, Quatela SE, Philips MR, Settleman J . Activated Kras, but not Hras or Nras, may initiate tumors of endodermal origin via stem cell expansion. Mol Cell Biol 2008; 28: 2659–2674. CASGoogle Scholar
- Mainardi S, Mijimolle N, Francoz S, Vicente-Dueñas C, Sánchez-García I, Barbacid M . Identification of cancer initiating cells in K-Ras driven lung adenocarcinoma. Proc Natl Acad Sci USA 2014; 111: 255–260. CASGoogle Scholar
- Xu X, Rock JR, Lu Y, Futtner C, Schwab B, Guinney J et al. Evidence for type II cells as cells of origin of K-Ras-induced distal lung adenocarcinoma. Proc Natl Acad Sci USA 2012; 109: 4910–4915. CASGoogle Scholar
- Lin C, Song H, Huang C, Yao E, Gacayan R, Xu SM et al. Alveolar type II cells possess the capability of initiating lung tumor development. PLoS One 2012; 7: e53817. CASGoogle Scholar
- Sutherland KD, Song JY, Kwon MC, Proost N, Zevenhoven J, Berns A . Multiple cells-of-origin of mutant K-Ras-induced mouse lung adenocarcinoma. Proc Natl Acad Sci USA 2014; 111: 4952–4957. CASGoogle Scholar
- Politi K, Zakowski MF, Fan PD, Schonfeld EA, Pao W, Varmus HE . Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev 2006; 20: 1496–1510. CASGoogle Scholar
- Curtis SJ, Sinkevicius KW, Li D, Lau AN, Roach RR, Zamponi R et al. Primary tumor genotype is an important determinant in identification of lung cancer propagating cells. Cell Stem Cell 2010; 7: 127–133. CASGoogle Scholar
- Tsai JH, Yang J . Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev 2013; 27: 2192–2206. CASGoogle Scholar
- Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet 2012; 44: 852–860. CASGoogle Scholar
- Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011; 3: 75ra26. Google Scholar
- Uramoto H, Shimokawa H, Hanagiri T, Kuwano M, Ono M . Expression of selected gene for acquired drug resistance to EGFR-TKI in lung adenocarcinoma. Lung Cancer 2011; 73: 361–365. Google Scholar
- Borczuk AC, Gorenstein L, Walter KL, Assaad AA, Wang L, Powell CA . Non-small-cell lung cancer molecular signatures recapitulate lung developmental pathways. Am J Pathol 2003; 163: 1949–1960. CASGoogle Scholar
- Watson WL, Berg JW . Oat cell lung cancer. Cancer 1962; 15: 759–768. CASGoogle Scholar
- Alam N, Gustafson KS, Ladanyi M, Zakowski MF, Kapoor A, Truskinovsky AM et al. Small-cell carcinoma with an epidermal growth factor receptor mutation in a never-smoker with gefitinib-responsive adenocarcinoma of the lung. Clin Lung Cancer 2010; 11: E1–E4. Google Scholar
- Popat S, Wotherspoon A, Nutting CM, Gonzalez D, Nicholson AG, O'Brien M . Transformation to "high grade" neuroendocrine carcinoma as an acquired drug resistance mechanism in EGFR-mutant lung adenocarcinoma. Lung Cancer 2013; 80: 1–4. CASGoogle Scholar
- Watanabe S, Sone T, Matsui T, Yamamura K, Tani M, Okazaki A et al. Transformation to small-cell lung cancer following treatment with EGFR tyrosine kinase inhibitors in a patient with lung adenocarcinoma. Lung Cancer 2013; 82: 370–372. Google Scholar
- Filosso PL, Ruffini E, Asioli S, Giobbe R, Macri L, Bruna MC et al. Adenosquamous lung carcinomas: a histologic subtype with poor prognosis. Lung Cancer 2011; 74: 25–29. Google Scholar
- Cooke DT, Nguyen DV, Yang Y, Chen SL, Yu C, Calhoun RF . Survival comparison of adenosquamous, squamous cell, and adenocarcinoma of the lung after lobectomy. Ann Thorac Surg 2010; 90: 943–948. Google Scholar
- Nakagawa K, Yasumitu T, Fukuhara K, Shiono H, Kikui M . Poor prognosis after lung resection for patients with adenosquamous carcinoma of the lung. Ann Thorac Surg 2003; 75: 1740–1744. Google Scholar
- Cheung WK, Zhao M, Liu Z, Stevens LE, Cao PD, Fang JE et al. Control of alveolar differentiation by the lineage transcription factors GATA6 and HOPX inhibits lung adenocarcinoma metastasis. Cancer Cell 2013; 23: 725–738. CASGoogle Scholar
- Cancer Genome Atlas Research N. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014; 511: 543–550. Google Scholar
- Han X, Li F, Fang Z, Gao Y, Li F, Fang R et al. Transdifferentiation of lung adenocarcinoma in mice with Lkb1 deficiency to squamous cell carcinoma. Nat Commun 2014; 5: 3261. Google Scholar
- Gao Y, Zhang W, Han X, Li F, Wang X, Wang R et al. YAP inhibits squamous transdifferentiation of Lkb1-deficient lung adenocarcinoma through ZEB2-dependent DNp63 repression. Nat Commun 2014; 5: 4629. CASGoogle Scholar
- Ischenko I, Liu J, Petrenko O, Hayman MJ . Transforming growth factor-beta signaling network regulates plasticity and lineage commitment of lung cancer cells. Cell Death Differ 2014; 21: 1218–1228. CASGoogle Scholar
- Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM et al. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell 2009; 17: 290–298. CASGoogle Scholar
- Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W et al. beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem 2003; 278: 40231–40238. CASGoogle Scholar
- Shu W, Guttentag S, Wang Z, Andl T, Ballard P, Lu MM et al. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol 2005; 283: 226–239. CASGoogle Scholar
- Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 2008; 455: 1069–1075. CASGoogle Scholar
- Stewart DJ . Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst 2014; 106: djt356. Google Scholar
- Reynolds SD, Zemke AC, Giangreco A, Brockway BL, Teisanu RM, Drake JA et al. Conditional stabilization of beta-catenin expands the pool of lung stem cells. Stem Cells 2008; 26: 1337–1346. CASGoogle Scholar
- Pacheco-Pinedo EC, Durham AC, Stewart KM, Goss AM, Lu MM, Demayo FJ et al. Wnt/β-catenin signaling accelerates mouse lung tumorigenesis by imposing an embryonic distal progenitor phenotype on lung epithelium. J Clin Invest 2011; 121: 1935–1945. CASGoogle Scholar
- Sánchez-Rivera FJ, Papagiannakopoulos T, Romero R, Tammela T, Bauer MR, Bhutkar A et al. Rapid modelling of cooperating genetic events in cancer through somatic genome editing. Nature 2014; 516: 428–431. Google Scholar
- Nguyen DX, Chiang AC, Zhang XH, Kim JY, Kris MG, Ladanyi M et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell 2009; 138: 51–62. CASGoogle Scholar
- Bleckmann A, Siam L, Klemm F, Rietkotter E, Wegner C, Kramer F et al. Nuclear LEF1/TCF4 correlate with poor prognosis but not with nuclear beta-catenin in cerebral metastasis of lung adenocarcinomas. Clin Exp Metastasis 2013; 30: 471–482. CASGoogle Scholar
- Maeda Y, Dave V, Whitsett JA . Transcriptional control of lung morphogenesis. Physiol Rev 2007; 87: 219–244. CASGoogle Scholar
- Burkhart DL, Sage J . Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer 2008; 8: 671–682. CASGoogle Scholar
- Wikenheiser-Brokamp KA . Rb family proteins differentially regulate distinct cell lineages during epithelial development. Development 2004; 131: 4299–4310. CASGoogle Scholar
- Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet 2009; 41: 1238–1242. CASGoogle Scholar
- Hussenet T, Dali S, Exinger J, Monga B, Jost B, Dembele D et al. SOX2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS One 2010; 5: e8960. Google Scholar
- Lu Y, Futtner C, Rock JR, Xu X, Whitworth W, Hogan BL et al. Evidence that SOX2 overexpression is oncogenic in the lung. PLoS One 2010; 5: e11022. Google Scholar
- Que J, Luo X, Schwartz RJ, Hogan BL . Multiple roles for Sox2 in the developing and adult mouse trachea. Development 2009; 136: 1899–1907. CASGoogle Scholar
- Mukhopadhyay A, Berrett KC, Kc U, Clair PM, Pop SM, Carr SR et al. Sox2 cooperates with Lkb1 loss in a mouse model of squamous cell lung cancer. Cell Rep 2014; 8: 40–49. CASGoogle Scholar
- Watanabe H, Ma Q, Peng S, Adelmant G, Swain D, Song W et al. SOX2 and p63 colocalize at genetic loci in squamous cell carcinomas. J Clin Invest 2014; 124: 1636–1645. CASGoogle Scholar
- Justilien V, Walsh MP, Ali SA, Thompson EA, Murray NR, Fields AP . The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate Hedgehog signaling in lung squamous cell carcinoma. Cancer Cell 2014; 25: 139–151. CASGoogle Scholar
- Xu X, Huang L, Futtner C, Schwab B, Rampersad RR, Lu Y et al. The cell of origin and subtype of K-Ras-induced lung tumors are modified by Notch and Sox2. Genes Dev 2014; 28: 1929–1939. CASGoogle Scholar
- Chang DR, Martinez Alanis D, Miller RK, Ji H, Akiyama H, McCrea PD et al. Lung epithelial branching program antagonizes alveolar differentiation. Proc Natl Acad Sci USA 2013; 110: 18042–18051. CASGoogle Scholar
- Rockich BE, Hrycaj SM, Shih HP, Nagy MS, Ferguson MA, Kopp JL et al. Sox9 plays multiple roles in the lung epithelium during branching morphogenesis. Proc Natl Acad Sci USA 2013; 110: E4456–E4464. CASGoogle Scholar
- Zhou CH, Ye LP, Ye SX, Li Y, Zhang XY, Xu XY et al. Clinical significance of SOX9 in human non-small cell lung cancer progression and overall patient survival. J Exp Clin Cancer Res 2012; 31: 18. Google Scholar
- Jiang SS, Fang WT, Hou YH, Huang SF, Yen BL, Chang JL et al. Upregulation of SOX9 in lung adenocarcinoma and its involvement in the regulation of cell growth and tumorigenicity. Clin Cancer Res 2010; 16: 4363–4373. CASGoogle Scholar
- Capaccione KM, Hong X, Morgan KM, Liu W, Bishop JM, Liu L et al. Sox9 mediates Notch1-induced mesenchymal features in lung adenocarcinoma. Oncotarget 2014; 5: 3636–3650. Google Scholar
- Ikeda K, Clark JC, Shaw-White JR, Stahlman MT, Boutell CJ, Whitsett JA . Gene structure and expression of human thyroid transcription factor-1 in respiratory epithelial cells. J Biol Chem 1995; 270: 8108–8114. CASGoogle Scholar
- Minoo P, Hamdan H, Bu D, Warburton D, Stepanik P, deLemos R . TTF-1 regulates lung epithelial morphogenesis. Dev Biol 1995; 172: 694–698. CASGoogle Scholar
- Weir BA, Woo MS, Getz G, Perner S, Ding L, Beroukhim R et al. Characterizing the cancer genome in lung adenocarcinoma. Nature 2007; 450: 893–898. CASGoogle Scholar
- Kendall J, Liu Q, Bakleh A, Krasnitz A, Nguyen KC, Lakshmi B et al. Oncogenic cooperation and coamplification of developmental transcription factor genes in lung cancer. Proc Natl Acad Sci USA 2007; 104: 16663–16668. CASGoogle Scholar
- Kwei KA, Kim YH, Girard L, Kao J, Pacyna-Gengelbach M, Salari K et al. Genomic profiling identifies TITF1 as a lineage-specific oncogene amplified in lung cancer. Oncogene 2008; 27: 3635–3640. CASGoogle Scholar
- Barletta JA, Perner S, Iafrate AJ, Yeap BY, Weir BA, Johnson LA et al. Clinical significance of TTF-1 protein expression and TTF-1 gene amplification in lung adenocarcinoma. J Cell Mol Med 2009; 13: 1977–1986. Google Scholar
- Tang X, Kadara H, Behrens C, Liu DD, Xiao Y, Rice D et al. Abnormalities of the TITF-1 lineage-specific oncogene in NSCLC: implications in lung cancer pathogenesis and prognosis. Clin Cancer Res 2011; 17: 2434–2443. CASGoogle Scholar
- Tanaka H, Yanagisawa K, Shinjo K, Taguchi A, Maeno K, Tomida S et al. Lineage-specific dependency of lung adenocarcinomas on the lung development regulator TTF-1. Cancer Res 2007; 67: 6007–6011. CASGoogle Scholar
- Yamaguchi T, Yanagisawa K, Sugiyama R, Hosono Y, Shimada Y, Arima C et al. NKX2-1/TITF1/TTF-1-Induced ROR1 is required to sustain EGFR survival signaling in lung adenocarcinoma. Cancer Cell 2012; 21: 348–361. CASGoogle Scholar
- Maeda Y, Tsuchiya T, Hao H, Tompkins DH, Xu Y, Mucenski ML et al. Kras(G12D) and Nkx2-1 haploinsufficiency induce mucinous adenocarcinoma of the lung. J Clin Invest 2012; 122: 4388–4400. CASGoogle Scholar
- Winslow MM, Dayton TL, Verhaak RG, Kim-Kiselak C, Snyder EL, Feldser DM et al. Suppression of lung adenocarcinoma progression by Nkx2-1. Nature 2011; 473: 101–104. CASGoogle Scholar
- Berghmans T, Paesmans M, Mascaux C, Martin B, Meert AP, Haller A et al. Thyroid transcription factor 1—a new prognostic factor in lung cancer: a meta-analysis. Ann Oncol 2006; 17: 1673–1676. CASGoogle Scholar
- Snyder EL, Watanabe H, Magendantz M, Hoersch S, Chen TA, Wang DG et al. Nkx2-1 represses a latent gastric differentiation program in lung adenocarcinoma. Mol Cell 2013; 50: 185–199. CASGoogle Scholar
- Yamaguchi T, Hosono Y, Yanagisawa K, Takahashi T . NKX2-1/TTF-1: an enigmatic oncogene that functions as a double-edged sword for cancer cell survival and progression. Cancer Cell 2013; 23: 718–723. CASGoogle Scholar
- Kim IJ, Quigley D, To MD, Pham P, Lin K, Jo B et al. Rewiring of human lung cell lineage and mitotic networks in lung adenocarcinomas. Nat Commun 2013; 4: 1701. Google Scholar
- Saito RA, Watabe T, Horiguchi K, Kohyama T, Saitoh M, Nagase T et al. Thyroid transcription factor-1 inhibits transforming growth factor-beta-mediated epithelial-to-mesenchymal transition in lung adenocarcinoma cells. Cancer Res 2009; 69: 2783–2791. CASGoogle Scholar
- Hosono Y, Yamaguchi T, Mizutani E, Yanagisawa K, Arima C, Tomida S et al. MYBPH, a transcriptional target of TTF-1, inhibits ROCK1, and reduces cell motility and metastasis. EMBO J 2012; 31: 481–493. CASGoogle Scholar
- Watanabe H, Francis JM, Woo MS, Etemad B, Lin W, Fries DF et al. Integrated cistromic and expression analysis of amplified NKX2-1 in lung adenocarcinoma identifies LMO3 as a functional transcriptional target. Genes Dev 2013; 27: 197–210. CASGoogle Scholar
- Molkentin JD . The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem 2000; 275: 38949–38952. CASGoogle Scholar
- Zhang Y, Goss AM, Cohen ED, Kadzik R, Lepore JJ, Muthukumaraswamy K et al. A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat Genet 2008; 40: 862–870. CASGoogle Scholar
- Yang H, Lu MM, Zhang L, Whitsett JA, Morrisey EE . GATA6 regulates differentiation of distal lung epithelium. Development 2002; 129: 2233–2246. CASGoogle Scholar
- Keijzer R, van Tuyl M, Meijers C, Post M, Tibboel D, Grosveld F et al. The transcription factor GATA6 is essential for branching morphogenesis and epithelial cell differentiation during fetal pulmonary development. Development 2001; 128: 503–511. CASGoogle Scholar
- Shaw-White JR, Bruno MD, Whitsett JA . GATA-6 activates transcription of thyroid transcription factor-1. J Biol Chem 1999; 274: 2658–2664. CASGoogle Scholar
- Liu C, Glasser SW, Wan H, Whitsett JA . GATA-6 and thyroid transcription factor-1 directly interact and regulate surfactant protein-C gene expression. J Biol Chem 2002; 277: 4519–4525. CASGoogle Scholar
- Bruno MD, Korfhagen TR, Liu C, Morrisey EE, Whitsett JA . GATA-6 activates transcription of surfactant protein A. J Biol Chem 2000; 275: 1043–1049. CASGoogle Scholar
- Kwei KA, Bashyam MD, Kao J, Ratheesh R, Reddy EC, Kim YH et al. Genomic profiling identifies GATA6 as a candidate oncogene amplified in pancreatobiliary cancer. PLoS Genet 2008; 4: e1000081. Google Scholar
- Zhong Y, Wang Z, Fu B, Pan F, Yachida S, Dhara M et al. GATA6 activates Wnt signaling in pancreatic cancer by negatively regulating the Wnt antagonist Dickkopf-1. PLoS One 2011; 6: e22129. CASGoogle Scholar
- Lin L, Bass AJ, Lockwood WW, Wang Z, Silvers AL, Thomas DG et al. Activation of GATA binding protein 6 (GATA6) sustains oncogenic lineage-survival in ;esophageal adenocarcinoma. Proc Natl Acad Sci USA 2012; 109: 4251–4256. CASGoogle Scholar
- Chia NY, Deng N, Das K, Huang D, Hu L, Zhu Y et al. Regulatory crosstalk between lineage-survival oncogenes KLF5, GATA4 and GATA6 cooperatively promotes gastric cancer development. Gut 2014, e-pub ahead of print 22 July 2014; doi:10.1136/gutjnl-2013-306596. Google Scholar
- Belaguli NS, Aftab M, Rigi M, Zhang M, Albo D, Berger DH . GATA6 promotes colon cancer cell invasion by regulating urokinase plasminogen activator gene expression. Neoplasia 2010; 12: 856–865. CASGoogle Scholar
- Capo-chichi CD, Roland IH, Vanderveer L, Bao R, Yamagata T, Hirai H et al. Anomalous expression of epithelial differentiation-determining GATA factors in ovarian tumorigenesis. Cancer Res 2003; 63: 4967–4977. CASGoogle Scholar
- Kamnasaran D, Qian B, Hawkins C, Stanford WL, Guha A . GATA6 is an astrocytoma tumor suppressor gene identified by gene trapping of mouse glioma model. Proc Natl Acad Sci USA 2007; 104: 8053–8058. CASGoogle Scholar
- Yang Y, Ahn YH, Gibbons DL, Zang Y, Lin W, Thilaganathan N et al. The Notch ligand Jagged2 promotes lung adenocarcinoma metastasis through a miR-200-dependent pathway in mice. J Clin Invest 2011; 121: 1373–1385. CASGoogle Scholar
- Guo M, Akiyama Y, House MG, Hooker CM, Heath E, Gabrielson E et al. Hypermethylation of the GATA genes in lung cancer. Clin Cancer Res 2004; 10: 7917–7924. CASGoogle Scholar
- Caslini C, Capo-chichi CD, Roland IH, Nicolas E, Yeung AT, Xu XX . Histone modifications silence the GATA transcription factor genes in ovarian cancer. Oncogene 2006; 25: 5446–5461. CASGoogle Scholar
- Yin Z, Gonzales L, Kolla V, Rath N, Zhang Y, Lu MM et al. Hop functions downstream of Nkx2.1 and GATA6 to mediate HDAC-dependent negative regulation of pulmonary gene expression. Am J Physiol Lung Cell Mol Physiol 2006; 291: L191–L199. CASGoogle Scholar
- Yamashita K, Kim MS, Park HL, Tokumaru Y, Osada M, Inoue H et al. HOP/OB1/NECC1 promoter DNA is frequently hypermethylated and involved in tumorigenic ability in esophageal squamous cell carcinoma. Mol Cancer Res 2008; 6: 31–41. CASGoogle Scholar
- Yamaguchi S, Asanoma K, Takao T, Kato K, Wake N . Homeobox gene HOPX is epigenetically silenced in human uterine endometrial cancer and suppresses estrogen-stimulated proliferation of cancer cells by inhibiting serum response factor. Int J Cancer 2009; 124: 2577–2588. CASGoogle Scholar
- Waraya M, Yamashita K, Katoh H, Ooki A, Kawamata H, Nishimiya H et al. Cancer specific promoter CpG Islands hypermethylation of HOP homeobox (HOPX) gene and its potential tumor suppressive role in pancreatic carcinogenesis. BMC Cancer 2012; 12: 397. CASGoogle Scholar
- Ooki A, Yamashita K, Kikuchi S, Sakuramoto S, Katada N, Kokubo K et al. Potential utility of HOP homeobox gene promoter methylation as a marker of tumor aggressiveness in gastric cancer. Oncogene 2010; 29: 3263–3275. CASGoogle Scholar
- Katoh H, Yamashita K, Waraya M, Margalit O, Ooki A, Tamaki H et al. Epigenetic silencing of HOPX promotes cancer progression in colorectal cancer. Neoplasia 2012; 14: 559–571. CASGoogle Scholar
- Chen Y, Yang L, Cui T, Pacyna-Gengelbach M, Petersen I . HOPX is methylated and exerts tumour suppressive function through Ras-induced senescence in human lung cancer. J Pathol 2015; 235: 397–407. CASGoogle Scholar
- Watanabe H, Meyerson M . Hopping between differentiation states in lung adenocarcinoma. Cancer Cell 2013; 23: 707–709. CASGoogle Scholar
- Martis PC, Whitsett JA, Xu Y, Perl AK, Wan H, Ikegami M . C/EBPalpha is required for lung maturation at birth. Development 2006; 133: 1155–1164. CASGoogle Scholar
- Basseres DS, Levantini E, Ji H, Monti S, Elf S, Dayaram T et al. Respiratory failure due to differentiation arrest and expansion of alveolar cells following lung-specific loss of the transcription factor C/EBPalpha in mice. Mol Cell Biol 2006; 26: 1109–1123. CASGoogle Scholar
- Sato A, Xu Y, Whitsett JA, Ikegami M . CCAAT/enhancer binding protein-α regulates the protease/antiprotease balance required for bronchiolar epithelium regeneration. Am J Respir Cell Mol Biol 2012; 47: 454–463. CASGoogle Scholar
- Costa DB, Dayaram T, D'Alo F, Wouters BJ, Tenen DG, Meyerson M et al. C/EBP alpha mutations in lung cancer. Lung Cancer 2006; 53: 253–254. Google Scholar
- Tada Y, Brena RM, Hackanson B, Morrison C, Otterson GA, Plass C . Epigenetic modulation of tumor suppressor CCAAT/enhancer binding protein alpha activity in lung cancer. J Natl Cancer Inst 2006; 98: 396–406. CASGoogle Scholar
- Halmos B, Huettner CS, Kocher O, Ferenczi K, Karp DD, Tenen DG . Down-regulation and antiproliferative role of C/EBPalpha in lung cancer. Cancer Res 2002; 62: 528–534. CASGoogle Scholar
- Sato A, Yamada N, Ogawa Y, Ikegami M . CCAAT/enhancer-binding protein-α suppresses lung tumor development in mice through the p38α MAP kinase pathway. PLoS One 2013; 8: e57013. CASGoogle Scholar
- Tomita T, Kido T, Kurotani R, Iemura S, Sterneck E, Natsume T et al. CAATT/enhancer-binding proteins alpha and delta interact with NKX2-1 to synergistically activate mouse secretoglobin 3A2 gene expression. J Biol Chem 2008; 283: 25617–25627. CASGoogle Scholar
- Wan H, Kaestner KH, Ang SL, Ikegami M, Finkelman FD, Stahlman MT et al. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development 2004; 131: 953–964. CASGoogle Scholar
- Wan H, Dingle S, Xu Y, Besnard V, Kaestner KH, Ang SL et al. Compensatory roles of Foxa1 and Foxa2 during lung morphogenesis. J Biol Chem 2005; 280: 13809–13816. CASGoogle Scholar
- Minoo P, Hu L, Xing Y, Zhu NL, Chen H, Li M et al. Physical and functional interactions between homeodomain NKX2.1 and winged helix/forkhead FOXA1 in lung epithelial cells. Mol Cell Biol 2007; 27: 2155–2165. CASGoogle Scholar
- Jimenez FR, Lewis JB, Belgique ST, Wood TT, Reynolds PR . Developmental lung expression and transcriptional regulation of claudin-6 by TTF-1, Gata-6, and FoxA2. Respir Res 2014; 15: 70. Google Scholar
- Halmos B, Bassères DS, Monti S, D'Aló F, Dayaram T, Ferenczi K et al. A transcriptional profiling study of CCAAT/enhancer binding protein targets identifies hepatocyte nuclear factor 3 beta as a novel tumor suppressor in lung cancer. Cancer Res 2004; 64: 4137–4147. CASGoogle Scholar
- Basseres DS, D'Alò F, Yeap BY, Löwenberg EC, Gonzalez DA, Yasuda H et al. Frequent downregulation of the transcription factor Foxa2 in lung cancer through epigenetic silencing. Lung Cancer 2012; 77: 31–37. Google Scholar
- Tang Y, Shu G, Yuan X, Jing N, Song J . FOXA2 functions as a suppressor of tumor metastasis by inhibition of epithelial-to-mesenchymal transition in human lung cancers. Cell Res 2011; 21: 316–326. CASGoogle Scholar
- Ito Y, Bae SC, Chuang LS . The RUNX family: developmental regulators in cancer. Nat Rev Cancer 2015; 15: 81–95. CASGoogle Scholar
- Lee KS, Lee YS, Lee JM, Ito K, Cinghu S, Kim JH et al. Runx3 is required for the differentiation of lung epithelial cells and suppression of lung cancer. Oncogene 2010; 29: 3349–3361. CASGoogle Scholar
- Lee JM, Shin JO, Cho KW, Hosoya A, Cho SW, Lee YS et al. Runx3 is a crucial regulator of alveolar differentiation and lung tumorigenesis in mice. Differentiation 2011; 81: 261–268. CASGoogle Scholar
- Liang Y, He L, Yuan H, Jin Y, Yao Y . Association between RUNX3 promoter methylation and non-small cell lung cancer: a meta-analysis. J Thorac Dis 2014; 6: 694–705. Google Scholar
- Yanagawa N, Tamura G, Oizumi H, Kanauchi N, Endoh M, Sadahiro M et al. Promoter hypermethylation of RASSF1A and RUNX3 genes as an independent prognostic prediction marker in surgically resected non-small cell lung cancers. Lung Cancer 2007; 58: 131–138. Google Scholar
- Zheng Y, Wang R, Song HZ, Pan BZ, Zhang YW, Chen LB . Epigenetic downregulation of RUNX3 by DNA methylation induces docetaxel chemoresistance in human lung adenocarcinoma cells by activation of the AKT pathway. Int J Biochem Cell Biol 2013; 45: 2369–2378. CASGoogle Scholar
- Fujii S, Ito K, Ito Y, Ochiai A . Enhancer of zeste homologue 2 (EZH2) down-regulates RUNX3 by increasing histone H3 methylation. J Biol Chem 2008; 283: 17324–17332. CASGoogle Scholar
- Chi XZ, Kim J, Lee YH, Lee JW, Lee KS, Wee H et al. Runt-related transcription factor RUNX3 is a target of MDM2-mediated ubiquitination. Cancer Res 2009; 69: 8111–8119. CASGoogle Scholar
- Lee YS, Lee JW, Jang JW, Chi XZ, Kim JH, Li YH et al. Runx3 inactivation is a crucial early event in the development of lung adenocarcinoma. Cancer Cell 2013; 24: 603–616. Google Scholar
- Okubo T, Knoepfler PS, Eisenman RN, Hogan BL . Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development 2005; 132: 1363–1374. CASGoogle Scholar
- Moens CB, Auerbach AB, Conlon RA, Joyner AL, Rossant J . A targeted mutation reveals a role for N-myc in branching morphogenesis in the embryonic mouse lung. Genes Dev 1992; 6: 691–704. CASGoogle Scholar
- Cohen JC, Scott DK, Miller J, Zhang J, Zhou P, Larson JE . Transient in utero knockout (TIUKO) of C-MYC affects late lung and intestinal development in the mouse. BMC Dev Biol 2004; 4: 4. Google Scholar
- Iwakawa R, Kohno T, Kato M, Shiraishi K, Tsuta K, Noguchi M et al. MYC amplification as a prognostic marker of early-stage lung adenocarcinoma identified by whole genome copy number analysis. Clin Cancer Res 2011; 17: 1481–1489. CASGoogle Scholar
- Iwakawa R, Takenaka M, Kohno T, Shimada Y, Totoki Y, Shibata T et al. Genome-wide identification of genes with amplification and/or fusion in small cell lung cancer. Genes Chromosomes Cancer 2013; 52: 802–816. CASGoogle Scholar
- Romero OA, Torres-Diz M, Pros E, Savola S, Gomez A, Moran S et al. MAX inactivation in small cell lung cancer disrupts MYC-SWI/SNF programs and is synthetic lethal with BRG1. Cancer Discov 2014; 4: 292–303. CASGoogle Scholar
- Tran PT, Fan AC, Bendapudi PK, Koh S, Komatsubara K, Chen J et al. Combined Inactivation of MYC and K-Ras oncogenes reverses tumorigenesis in lung adenocarcinomas and lymphomas. PLoS One 2008; 3: e2125. Google Scholar
- Allen TD, Zhu CQ, Jones KD, Yanagawa N, Tsao MS, Bishop JM . Interaction between MYC and MCL1 in the genesis and outcome of non-small-cell lung cancer. Cancer Res 2011; 71: 2212–2221. CASGoogle Scholar
- Allen TD, Rodriguez EM, Jones KD, Bishop JM . Activated Notch1 induces lung adenomas in mice and cooperates with Myc in the generation of lung adenocarcinoma. Cancer Res 2011; 71: 6010–6018. CASGoogle Scholar
- Soucek L, Whitfield JR, Sodir NM, Massó-Vallés D, Serrano E, Karnezis AN et al. Inhibition of Myc family proteins eradicates KRas-driven lung cancer in mice. Genes Dev 2013; 27: 504–513. CASGoogle Scholar
- Juan J, Muraguchi T, Iezza G, Sears RC, McMahon M . Diminished WNT -> β-catenin -> c-MYC signaling is a barrier for malignant progression of BRAFV600E-induced lung tumors. Genes Dev 2014; 28: 561–575. CASGoogle Scholar
- Adhikary S, Eilers M . Transcriptional regulation and transformation by Myc proteins. Nat Rev Mol Cell Biol 2005; 6: 635–645. CASGoogle Scholar
- Lin CY, Lovén J, Rahl PB, Paranal RM, Burge CB, Bradner JE et al. Transcriptional amplification in tumor cells with elevated c-Myc. Cell 2012; 151: 56–67. CASGoogle Scholar
- Sinha S, Adler AS, Field Y, Chang HY, Segal E . Systematic functional characterization of cis-regulatory motifs in human core promoters. Genome Res 2008; 18: 477–488. CASGoogle Scholar
- Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, Chang HY . Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell 2008; 2: 333–344. CASGoogle Scholar
- Rapp UR, Korn C, Ceteci F, Karreman C, Luetkenhaus K, Serafin V et al. MYC is a metastasis gene for non-small-cell lung cancer. PLoS One 2009; 4: e6029. Google Scholar
- Medina PP, Romero OA, Kohno T, Montuenga LM, Pio R, Yokota J et al. Frequent BRG1/SMARCA4-inactivating mutations in human lung cancer cell lines. Hum Mutat 2008; 29: 617–622. CASGoogle Scholar
- Wilson BG, Roberts CW . SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer 2011; 11: 481–492. CASGoogle Scholar
- Brabletz T . To differentiate or not—routes towards metastasis. Nat Rev Cancer 2012; 12: 425–436. CASGoogle Scholar
- Gibbons DL, Lin W, Creighton CJ, Rizvi ZH, Gregory PA, Goodall GJ et al. Contextual extracellular cues promote tumor cell EMT and metastasis by regulating miR-200 family expression. Genes Dev 2009; 23: 2140–2151. CASGoogle Scholar
- Brabletz T . EMT and MET in metastasis: where are the cancer stem cells? Cancer Cell 2012; 22: 699–701. CASGoogle Scholar
- Puisieux A, Brabletz T, Caramel J . Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol 2014; 16: 488–494. CASGoogle Scholar
- Tsuji T, Ibaragi S, Hu GF . Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Res 2009; 69: 7135–7139. CASGoogle Scholar
- Celià-Terrassa T, Meca-Cortés O, Mateo F, de Paz AM, Rubio N, Arnal-Estapé A et al. Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J Clin Invest 2012; 122: 1849–1868. Google Scholar
- Calbo J, van Montfort E, Proost N, van Drunen E, Beverloo HB, Meuwissen R et al. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell 2011; 19: 244–256. CASGoogle Scholar
- Cheung KJ, Gabrielson E, Werb Z, Ewald AJ . Collective invasion in breast cancer requires a conserved basal epithelial program. Cell 2013; 155: 1639–1651. CASGoogle Scholar
- Govaere O, Komuta M, Berkers J, Spee B, Janssen C, de Luca F et al. Keratin 19: a key role player in the invasion of human hepatocellular carcinomas. Gut 2014; 63: 674–685. CASGoogle Scholar
- Reynolds CP, Lemons RS . Retinoid therapy of childhood cancer. Hematol Oncol Clin North Am 2001; 15: 867–910. CASGoogle Scholar
- Saez-Ayala M, Montenegro MF, Sanchez-Del-Campo L, Fernandez-Perez MP, Chazarra S, Freter R et al. Directed phenotype switching as an effective antimelanoma strategy. Cancer Cell 2013; 24: 105–119. CASGoogle Scholar
- Konieczkowski DJ, Johannessen CM, Abudayyeh O, Kim JW, Cooper ZA, Piris A et al. A melanoma cell state distinction influences sensitivity to MAPK pathway inhibitors. Cancer Discov 2014; 4: 816–827. CASGoogle Scholar
- Rohle D, Popovici-Muller J, Palaskas N, Turcan S, Grommes C, Campos C et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 2013; 340: 626–630. CASGoogle Scholar
- Campos B, Wan F, Farhadi M, Ernst A, Zeppernick F, Tagscherer KE et al. Differentiation therapy exerts antitumor effects on stem-like glioma cells. Clin Cancer Res 2010; 16: 2715–2728. CASGoogle Scholar
- Yan M, Zhang Y, He B, Xiang J, Wang ZF, Zheng FM et al. IKKalpha restoration via EZH2 suppression induces nasopharyngeal carcinoma differentiation. Nat Commun 2014; 5: 3661. CASGoogle Scholar
- Tremblay AM, Missiaglia E, Galli GG, Hettmer S, Urcia R, Carrara M et al. The Hippo transducer YAP1 transforms activated satellite cells and is a potent effector of embryonal rhabdomyosarcoma formation. Cancer Cell 2014; 26: 273–287. CASGoogle Scholar
- Lockwood WW, Zejnullahu K, Bradner JE, Varmus H . Sensitivity of human lung adenocarcinoma cell lines to targeted inhibition of BET epigenetic signaling proteins. Proc Natl Acad Sci U S A 2012; 109: 19408–19413. CASGoogle Scholar
- Shimamura T, Chen Z, Soucheray M, Carretero J, Kikuchi E, Tchaicha JH et al. Efficacy of BET bromodomain inhibition in Kras-mutant non-small cell lung cancer. Clin Cancer Res 2013; 19: 6183–6192. CASGoogle Scholar
- Kwiatkowski N, Zhang T, Rahl PB, Abraham BJ, Reddy J, Ficarro SB et al. Targeting transcription regulation in cancer with a covalent CDK7 inhibitor. Nature 2014; 511: 616–620. CASGoogle Scholar
- Chipumuro E, Marco E, Christensen CL, Kwiatkowski N, Zhang T, Hatheway CM et al. CDK7 inhibition suppresses super-enhancer-linked oncogenic transcription in MYCN-driven cancer. Cell 2014; 159: 1126–1139. CASGoogle Scholar
- Christensen CL, Kwiatkowski N, Abraham BJ, Carretero J, Al-Shahrour F, Zhang T et al. Targeting transcriptional addictions in small cell lung cancer with a covalent CDK7 inhibitor. Cancer Cell 2014; 26: 909–922. CASGoogle Scholar
- Corcoran RB, Cheng KA, Hata AN, Faber AC, Ebi H, Coffee EM et al. Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer Cell 2013; 23: 121–128. CASGoogle Scholar
- Byers LA, Diao L, Wang J, Saintigny P, Girard L, Peyton M et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res 2013; 19: 279–290. CASGoogle Scholar
Acknowledgements
We thank all members of the Nguyen laboratory for their discussions and critical reviews of the manuscript. We apologize for omitting some examples and primary references due to space constraints. Our research is funded by grants from Uniting Against Lung Cancer (to WKCC) and the National Cancer Institute (1R01CA166376, 1R21CA170537 and 1R01CA191489 to DXN).







